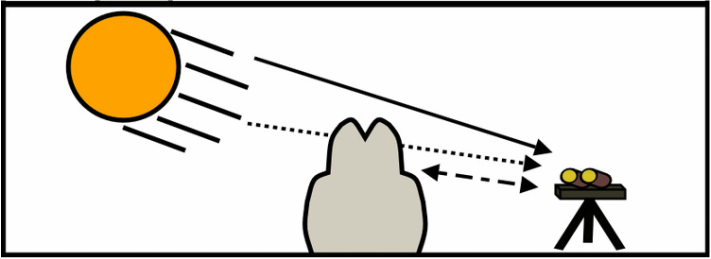
 Ultraviolet cameras set up on the flanks of Mt. Etna. Ultraviolet cameras set up on the flanks of Mt. Etna. I have realised that I often talk about my work using UV (Ultraviolet) cameras on this blog but I have never properly explained how they work and why we specifically use them! So here I go. Monitoring volcanic gas release is one of the major things volcanologists can do to aid with eruption forecasting (see this recent post by James Hickey to understand why we call it forecasting). A number of techniques have therefore been developed and used to measure the release of volcanic gases from volcanoes. One of such techniques is the UV camera (Bluth et al. 2007; Mori and Burton, 2006), pictured on the right. These were developed to solve the time and spatial issues with previous techniques (e.g., DOAS and COSPEC). These previous techniques required building up a plume profile using a stepper motor to scan across a plume or traversing under it. Traversing could be conducted on foot, by moped, by car, or aircraft! The UV camera immediately solves this problem as it essentially works like a conventional camera by taking still images using the UV part of the electromagnetic spectrum, instead of visible light like your everyday camera. The UV camera and previous techniques all work on the principle of absorption of UV light by the volcanic gas sulphur dioxide (SO2). We specifically use SO2 due to the very low atmospheric background concentration of this gas. This makes it very easy to resolve the volcanic component. We can then, using two different cameras taking images at exactly the same time, compare two images (captured at the same time from each camera) where SO2 does and doesn't absorb (310 and 330 nm respectively). The graphic below illustrates the basic concept.  The plume from the NEC at Mt. Etna. Lines (a) and (b) indicate locations used to calculate plume transport speed. The plume from the NEC at Mt. Etna. Lines (a) and (b) indicate locations used to calculate plume transport speed. We can then use the Lambert-Beer law to combine the images captured at each wavelength to work out the strength of absorption in each pixel. At this stage the calculated value will preserve real fluctuations in concentration but we won't know exact SO2 values without calibration! There are two distinct ways of doing this. The first method is to image quartz gas cells, with known concentrations of SO2, with both cameras. We can then use the Lambert-Beer law again to combine the images at the separate wavelengths (310 and 330 nm - where SO2 does and doesn't absorb), which will create a series of new images with the absorption strength in each pixel - but this time we know exactly how much gas should be in front of the image - we can therefore use this to create what we call a calibration curve (or line) to map the values in the image of the volcanic plume to real SO2 concentrations. The second method is to combine the DOAS method (Differential Optical Absorption Spectroscopy) with the UV camera images. DOAS works in a different way, essentially measuring a single pixel in the plume, and it has the ability to determine actual SO2 concentration (based on the absorption structure of SO2 at different wavelengths). During acquisition the concentration of SO2 will change at the location the DOAS is pointing, again enabling us to calibrate our images through comparison of the absorption pixel values in the UV camera images for the same pixel with the known value. Both of these processes are illustrated in the photo slideshow below. We can then calculate the rate at which SO2 is released from a specific crater, vent or fumarole by multiplying the plume speed by the total concentration of SO2 contained along a single line (e.g., line [a] in the image above), what we call the integrated column amount. Plume speed can be determined in a couple ways, including: optical flow algorithms which map the movement of pixels from image to image (e.g., Peters et al. 2015) or by using a cross-correlation technique (e.g., McGonigle et al. 2005) which uses the structure of the gas plume and how long it takes for parts of the plume to move from one point to another (illustrated in picture of plume from Etna above as lines [a] and [b]). So this how we make UV camera measurements! The frequency that images can be taken is generally every 1 second but this can potentially be increased to 15 or even 30. This has the obvious benefit of being able to measure rapidly changing volcanic gas emissions associated with explosive activity such as strombolian volcanism or during passive activity (the constant quiescent release of gas). We can also begin to compare SO2 flux measurements with other datasets collected at similar time frequencies (e.g., Burton et al. 2015 offer an overview of UV camera studies). The benefits offered with an increase in spatial resolution can allow us to image more than one source at the same time - perfect where more than one crater (e.g., Etna) may be emitting gases or we may be observing a number of fumaroles (e.g., Vulcano). I hope this has been interesting aside into one of the major pieces of equipment which allows me to do my research! My next post will be all about a low cost UV camera system developed at the University of Sheffield and recently published in the Journal - Sensors. References
Comments are closed.
|
Archives
July 2023
|
 RSS Feed
RSS Feed